Additional Information
| Specialty | |
|---|---|
| Manufacturer | |
| Brand |
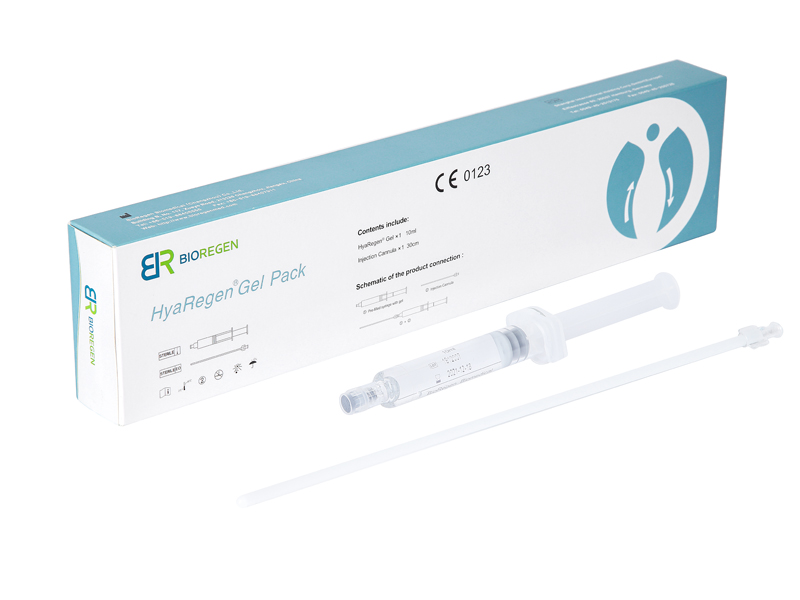
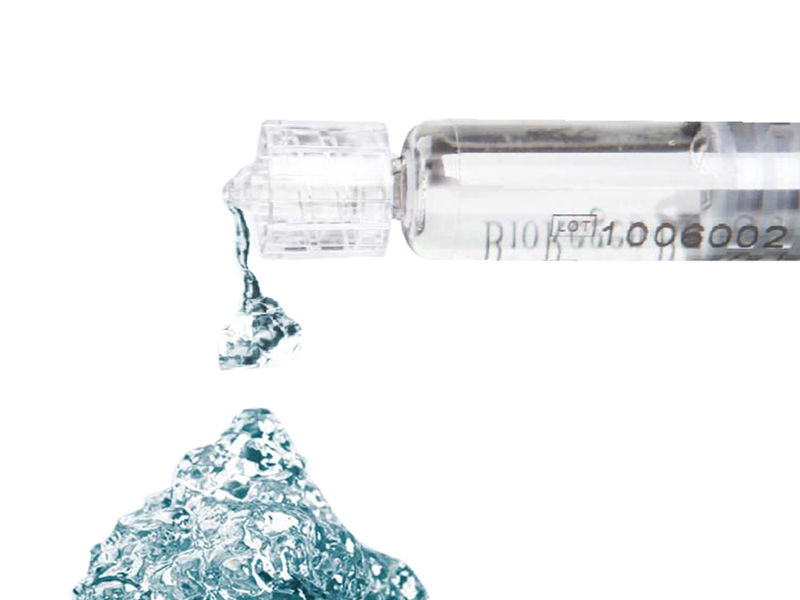
HyaRegen Gel is a sterile, transparent and highly viscous gel. The active component is cross-linked molecules of hyaluronan (HA) from a non-animal source.
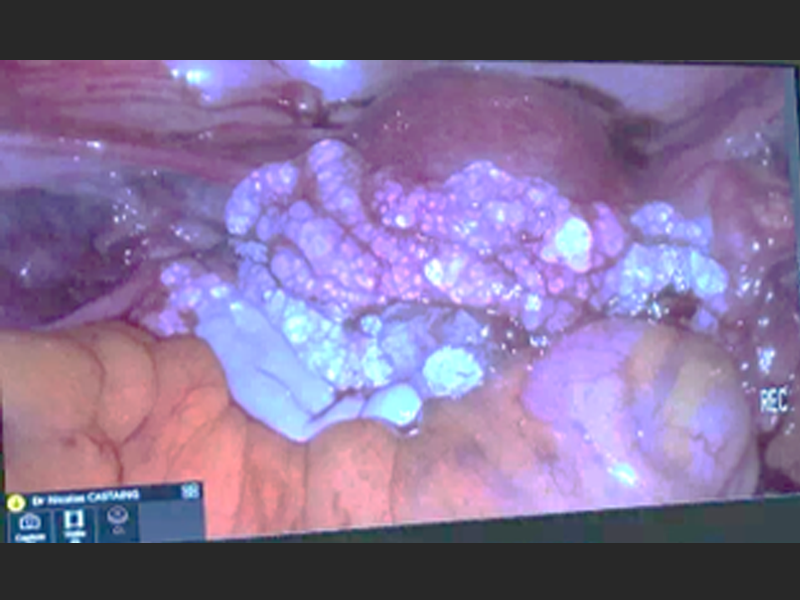
HyaRegen Gel is indicated for the prevention or reduction of post-surgical adhesion formation in the abdominopelvic area after laparoscopic/hysteroscopic and open gynaecological surgical procedures, e.g. ovary surgeries, fallopian tube surgeries, myomectomy, surgeries for endometriosis, adhesiolysis etc. 1-4.
| Self-crosslinking technology, protected by 44 international patents. Easy to transport, store under room temperature and operate | Jelly-like gel, sustained isolation and accelerated healing of pelvic trauma and surrounding tissue surfaces within 7-14 days after surgery ¹-6. | Can effectively prevent pelvic adhesion, improve postoperative quality of life, and inhibit tumour cell growth, metastasis and recurrence, proven by studies ¹-6. |
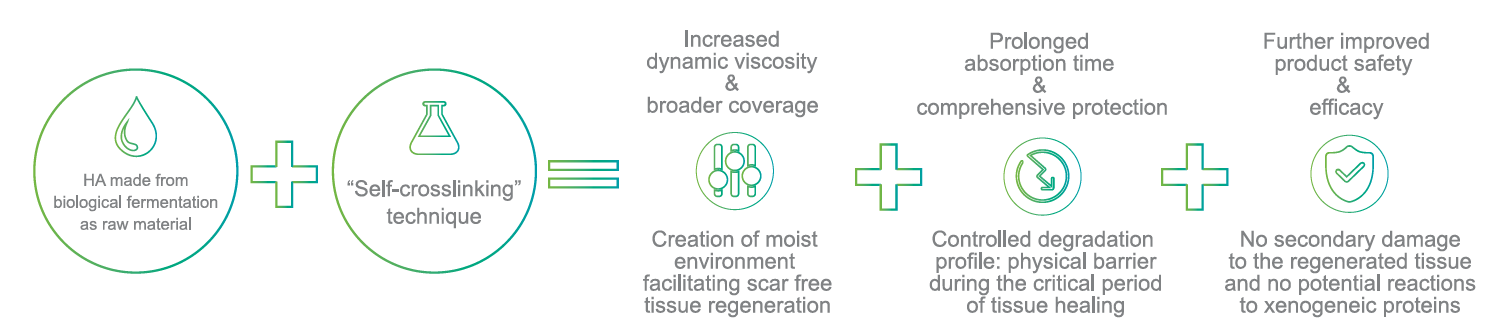
Optimised and controlled degradation of the Gel ensures coverage and protection of the organs.

HyaRegen Gel degrades in the abdominopelvic cavity for up to 14 days. 1-6
References
1. De Wilde, Rudy Leon, Rajesh Devassy, Richard P. G. ten Broek, Charles E. Miller, Aizura Adlan, Prudence Aquino, Sven Becker, Ferry Darmawan, Marco Gergolet, Maria Antonia E. Habana, Chong Kiat Khoo, Philippe R. Koninckx, Matthias Korell, Harald Krentel, Olarik Musigavong, George Pistofidis, Shailesh Puntambekar, Ichnandy A. Rachman, Fatih Sendag, Markus Wallwiener, and Luz Angela Torres-de la Roche. 2022. “The Future of Adhesion Prophylaxis Trials in Abdominal Surgery: An Expert Global Consensus” Journal of Clinical Medicine 11, no. 6: 1476. https://doi.org/10.3390/jcm11061476
2. De Wilde RL, Brölmann H, Koninckx PR, Lundorff P, Lower AM, Wattiez A, Mara M, Wallwiener M; The Anti-Adhesions in Gynecology Expert Panel (ANGEL). Prevention of adhesions in gynaecological surgery: the 2012 European field guideline. Gynecol Surg. 2012 Nov;9(4):365-368. doi: 10.1007/s10397-012-0764-2. Epub 2012 Aug 24. PMID: 23144639; PMCID: PMC3491197.
3. Liu C, Lu Q, Zhang Z, Xue M, Zhang Y, Zhang Y, Wang H, Li H, Zhou Y, Zhang Z, Li W; HyaRegen Adhesion Study Group. A Randomized Controlled Trial on the Efficacy and Safety of a New Crosslinked Hyaluronan Gel in Reducing Adhesions after Gynecologic Laparoscopic Surgeries. J Minim Invasive Gynecol. 2015 Jul-Aug;22(5):853-63. doi: 10.1016/j.jmig.2015.04.011. Epub 2015 Apr 20. PMID: 25906706.
4. Ekin M, Kaya C, Erdoğan ŞV, Bahçeci E, Baghaki S, Yaşar L. The effect of new cross linked hyaluronan gel on quality of life of patients after deep infiltrating endometriosis surgery: a randomized controlled pilot study. J Obstet Gynaecol. 2021 Feb;41(2):263-268. doi: 10.1080/01443615.2020.1755628. Epub 2020 Jun 12. PMID: 32530335.
5. Xiao S. et al.: Giorn. It. Ost. Gin. 2015; XXXVII:4.
6. KUTLU, Tayfun & Özkaya, Enis & ŞANVERDİ, a. (2018). Using New-Crosslinked Hyaluronic Acid Gel to Prevent Intrauterine Adhesion After Hysteroscopic Septum Resection: A Prospective, Randomized, Controlled Study. Türk Üreme Tıbbı ve Cerrahisi Dergisi. 2. 43-46. 10.24074/tjrms.2018-61186.
| Specialty | |
|---|---|
| Manufacturer | |
| Brand |
The information contained within this website is designed and intended for healthcare professionals only.
Endotherapeutics will not be liable for any actions taken in reliance of the information contained within the website. The information contained within the website does not constitute medical advice.
If you are a patient who requires treatment or management of a medical condition, please click No and you will be redirected to Endo Personal Care.